The first and only study to measure patient-reported TD impact using multiple validated scales
Data on file. Neurocrine Biosciences, Inc.
This website is intended for US healthcare professionals only.
Review tardive dyskinesia (TD) movements across a broad range of adult patients and see the effect of treatment with INGREZZA® (valbenazine) capsules.
Real-world cases feature patients treated with INGREZZA in clinical practice, and not from the INGREZZA clinical trial program. Treatment decisions were based on healthcare providers’ clinical judgment.
These patients were compensated by Neurocrine Biosciences, Inc., and have consented to Neurocrine’s use of the videos and their protected health information.

Major depressive disorder



Note: Not all movements may be visible in these videos

Schizoaffective disorder and depression
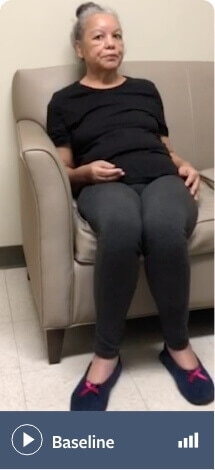

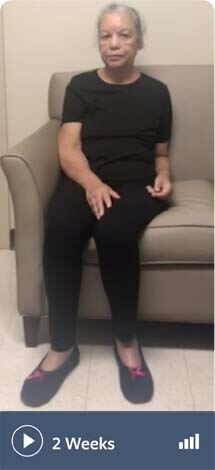

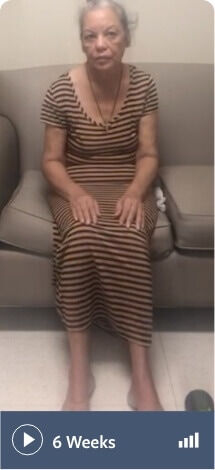
Note: Not all movements may be visible in these videos

Schizoaffective disorder (bipolar type) and unspecified anxiety disorder
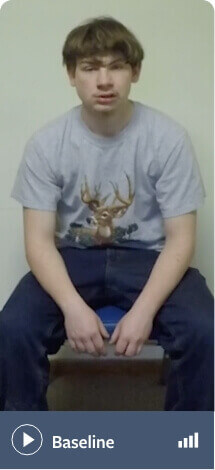




Note: Not all movements may be visible in these videos

Schizophrenia



Note: Not all movements may be visible in these videos

Schizoaffective disorder



Note: Not all movements may be visible in these videos

Bipolar disorder
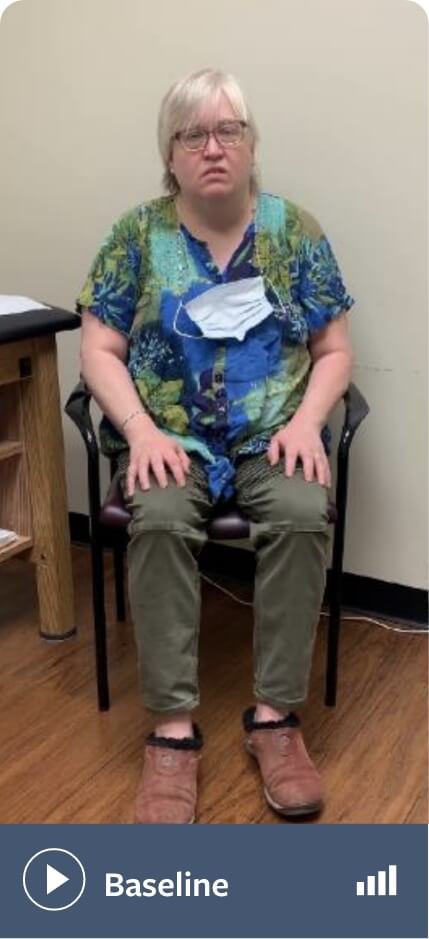


Note: Not all movements may be visible in these videos
The only VMAT2 inhibitor that offers an effective starting dosage you can adjust based on response and tolerability1
EXPLORE DOSINGExplore how INGREZZA reduced TD severity in both short- and long-term studies of adult patients with TD
VISIT EFFICACY IN TDRequest an office visit from a Neurocrine representative for additional information, patient resources, or samples of INGREZZA
REQUEST INFOINGREZZA® (valbenazine) capsules and INGREZZA® SPRINKLE (valbenazine) capsules are indicated in adults for the treatment of tardive dyskinesia and for the treatment of chorea associated with Huntington’s disease.
Depression and Suicidality in Patients with Huntington’s Disease: VMAT2 inhibitors, including INGREZZA and INGREZZA SPRINKLE, can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidal ideation, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidal ideation and behavior and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation, which are increased in frequency in patients with Huntington’s disease.
INGREZZA and INGREZZA SPRINKLE are contraindicated in patients with a history of hypersensitivity to valbenazine or any components of INGREZZA or INGREZZA SPRINKLE.
Hypersensitivity reactions, including cases of angioedema involving the larynx, glottis, lips, and eyelids, have been reported in patients after taking the first or subsequent doses of INGREZZA. Angioedema associated with laryngeal edema can be fatal. If any of these reactions occur, discontinue INGREZZA or INGREZZA SPRINKLE.
INGREZZA and INGREZZA SPRINKLE can cause somnolence and sedation. Patients should not perform activities requiring mental alertness such as operating a motor vehicle or operating hazardous machinery until they know how they will be affected by INGREZZA or INGREZZA SPRINKLE.
INGREZZA and INGREZZA SPRINKLE may prolong the QT interval, although the degree of QT prolongation is not clinically significant at concentrations expected with recommended dosing. INGREZZA and INGREZZA SPRINKLE should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, assess the QT interval before increasing the dosage.
A potentially fatal symptom complex referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with drugs that reduce dopaminergic transmission, including INGREZZA. The management of NMS should include immediate discontinuation of INGREZZA or INGREZZA SPRINKLE, intensive symptomatic treatment and medical monitoring, and treatment of any concomitant serious medical problems. If treatment with INGREZZA or INGREZZA SPRINKLE is needed after recovery from NMS, patients should be monitored for signs of recurrence.
INGREZZA and INGREZZA SPRINKLE may cause parkinsonism. Parkinsonism has also been observed with other VMAT2 inhibitors. Reduce the dose or discontinue INGREZZA or INGREZZA SPRINKLE treatment in patients who develop clinically significant parkinson-like signs or symptoms.
The most common adverse reaction in patients with tardive dyskinesia (≥5% and twice the rate of placebo) is somnolence.
The most common adverse reactions in patients with chorea associated with Huntington’s disease (≥5% and twice the rate of placebo) are somnolence/lethargy/sedation, urticaria, rash, and insomnia.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit MedWatch at www.fda.gov/medwatch or call 1-800-FDA-1088.
Dosage Forms and Strengths: INGREZZA and INGREZZA SPRINKLE are available in 40 mg, 60 mg, and 80 mg capsules.
Please see full Prescribing Information, including Boxed Warning.
Depression and Suicidality in Patients with Huntington’s Disease: VMAT2 inhibitors, including INGREZZA, can increase the risk of depression and suicidal thoughts and
INGREZZA® (valbenazine) capsules and INGREZZA® SPRINKLE (valbenazine) capsules are indicated in adults for the treatment of tardive dyskinesia and for the treatment of chorea associated with Huntington’s disease.
Depression and Suicidality in Patients with Huntington’s Disease: VMAT2 inhibitors, including INGREZZA and INGREZZA SPRINKLE, can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidal ideation, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidal ideation and behavior and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation, which are increased in frequency in patients with Huntington’s disease.
INGREZZA® (valbenazine) capsules and INGREZZA® SPRINKLE (valbenazine) capsules are indicated in adults for the treatment of tardive dyskinesia and for the treatment of chorea associated with Huntington’s disease.
Depression and Suicidality in Patients with Huntington’s Disease: VMAT2 inhibitors, including INGREZZA and INGREZZA SPRINKLE, can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidal ideation, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidal ideation and behavior and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation, which are increased in frequency in patients with Huntington’s disease.
INGREZZA and INGREZZA SPRINKLE are contraindicated in patients with a history of hypersensitivity to valbenazine or any components of INGREZZA or INGREZZA SPRINKLE.
Hypersensitivity reactions, including cases of angioedema involving the larynx, glottis, lips, and eyelids, have been reported in patients after taking the first or subsequent doses of INGREZZA. Angioedema associated with laryngeal edema can be fatal. If any of these reactions occur, discontinue INGREZZA or INGREZZA SPRINKLE.
INGREZZA and INGREZZA SPRINKLE can cause somnolence and sedation. Patients should not perform activities requiring mental alertness such as operating a motor vehicle or operating hazardous machinery until they know how they will be affected by INGREZZA or INGREZZA SPRINKLE.
INGREZZA and INGREZZA SPRINKLE may prolong the QT interval, although the degree of QT prolongation is not clinically significant at concentrations expected with recommended dosing. INGREZZA and INGREZZA SPRINKLE should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, assess the QT interval before increasing the dosage.
A potentially fatal symptom complex referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with drugs that reduce dopaminergic transmission, including INGREZZA. The management of NMS should include immediate discontinuation of INGREZZA or INGREZZA SPRINKLE, intensive symptomatic treatment and medical monitoring, and treatment of any concomitant serious medical problems. If treatment with INGREZZA or INGREZZA SPRINKLE is needed after recovery from NMS, patients should be monitored for signs of recurrence.
INGREZZA and INGREZZA SPRINKLE may cause parkinsonism. Parkinsonism has also been observed with other VMAT2 inhibitors. Reduce the dose or discontinue INGREZZA or INGREZZA SPRINKLE treatment in patients who develop clinically significant parkinson-like signs or symptoms.
The most common adverse reaction in patients with tardive dyskinesia (≥5% and twice the rate of placebo) is somnolence.
The most common adverse reactions in patients with chorea associated with Huntington’s disease (≥5% and twice the rate of placebo) are somnolence/lethargy/sedation, urticaria, rash, and insomnia.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit MedWatch at www.fda.gov/medwatch or call 1-800-FDA-1088.
Dosage Forms and Strengths: INGREZZA and INGREZZA SPRINKLE are available in 40 mg, 60 mg, and 80 mg capsules.
Please see full Prescribing Information, including Boxed Warning.