Mechanism of action and pharmacology of INGREZZA
INGREZZA is a uniquely selective VMAT2 inhibitor with one-capsule, once-daily dosing1,2
Tardive dyskinesia (TD)
Tardive dyskinesia
Huntington's disease (HD) Chorea
HD chorea
TD—an often persistent movement disorder induced by prolonged DRBA exposure4
-
1
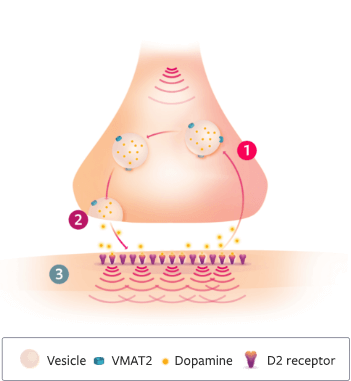
VMAT2 plays a key role in dopamine signaling2
- VMAT2 is a transporter protein found in presynaptic neurons of the CNS
- VMAT2 packages monoamines (eg, dopamine) for release into the synaptic cleft
- 2 TD is associated with prolonged exposure to DRBAs, including antipsychotics4
- 3 Prolonged exposure to DRBAs causes hypersensitivity in postsynaptic dopamine D2 receptors in areas that control motor function2
CNS, central nervous system; DRBA, dopamine receptor blocking agent; VMAT2, vesicular monoamine transporter 2. |
While the MOA of INGREZZA® (valbenazine) capsules is unclear, it is believed:
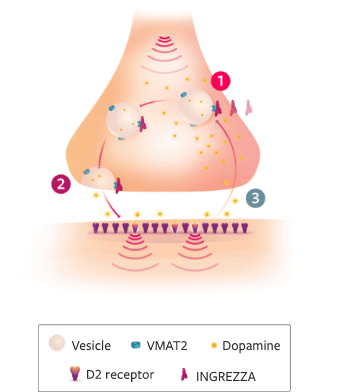
- 1It may be mediated through selective inhibition of VMAT2 in presynaptic neurons, with no appreciable binding affinity for VMAT1, dopaminergic receptors, or serotonergic receptors2
- 2INGREZZA is believed to provide reversible reductions of dopamine release into the synaptic cleft2
- 3INGREZZA is believed to reduce the amount of dopamine available to hypersensitive postsynaptic dopamine D2 receptors1,2
CNS, central nervous system; DRBA, dopamine receptor blocking agent; VMAT2, vesicular monoamine transporter 2. |
Neurodegeneration in the striatum leads to increased dopamine signaling that results in chorea5
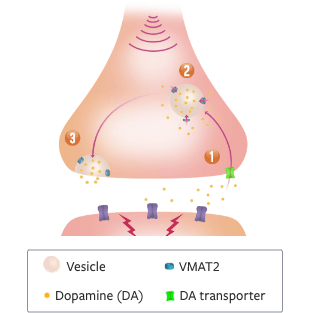
DOPAMINE SIGNALING CYCLE:
- 1 Dopamine is taken up into the cytosol of the presynaptic neuron through the DA transporter5
- 2 Here, it is packaged into vesicles by VMAT2 or quickly broken down by enzymes5
- 3 When the neuron is activated, vesicles full of dopamine fuse with the presynaptic membrane and dopamine is released into the synapse6
VMAT2, vesicular monoamine transporter 2. |
Only INGREZZA® (valbenazine) capsules selectively inhibits VMAT2 to counteract increased dopamine signaling7,8
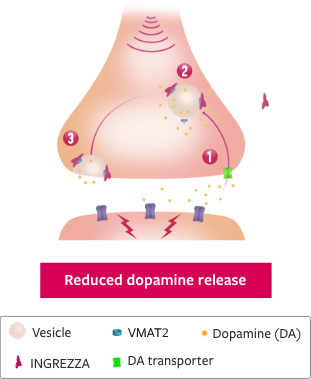
HYPOTHETICAL DOPAMINE SIGNALING CYCLE WITH INGREZZA9
While the mechanism of action of INGREZZA is unclear, it is believed that:
- 1 Dopamine is taken up into the cytosol of the presynaptic neuron through the DA transporter
- 2 With INGREZZA blocking VMAT2, less dopamine is packaged into vesicles, and free dopamine is quickly broken down into the cytosol
- 3 When the neuron is activated, vesicles with dopamine fuse with the presynaptic membrane, releasing dopamine into the synapse
VMAT2, vesicular monoamine transporter 2. |
ONLY INGREZZA IS UNIQUELY SELECTIVE FOR VMAT2
When all you want is VMAT2 inhibition, all you need is INGREZZA. Only INGREZZA exclusively delivers one primary metabolite (+ α) for potent and selective inhibition of VMAT21-3,*
View full transcript
| * | Based on in vitro VMAT2 binding affinity of dihydrotetrabenazine (HTBZ) metabolites and the primary active metabolite of INGREZZA, + α HTBZ. The clinical significance of in vitro data is unknown and is not meant to imply clinical outcomes. |
Unlike other VMAT2 inhibitors, INGREZZA delivers only the selective + α metabolite3,7,8
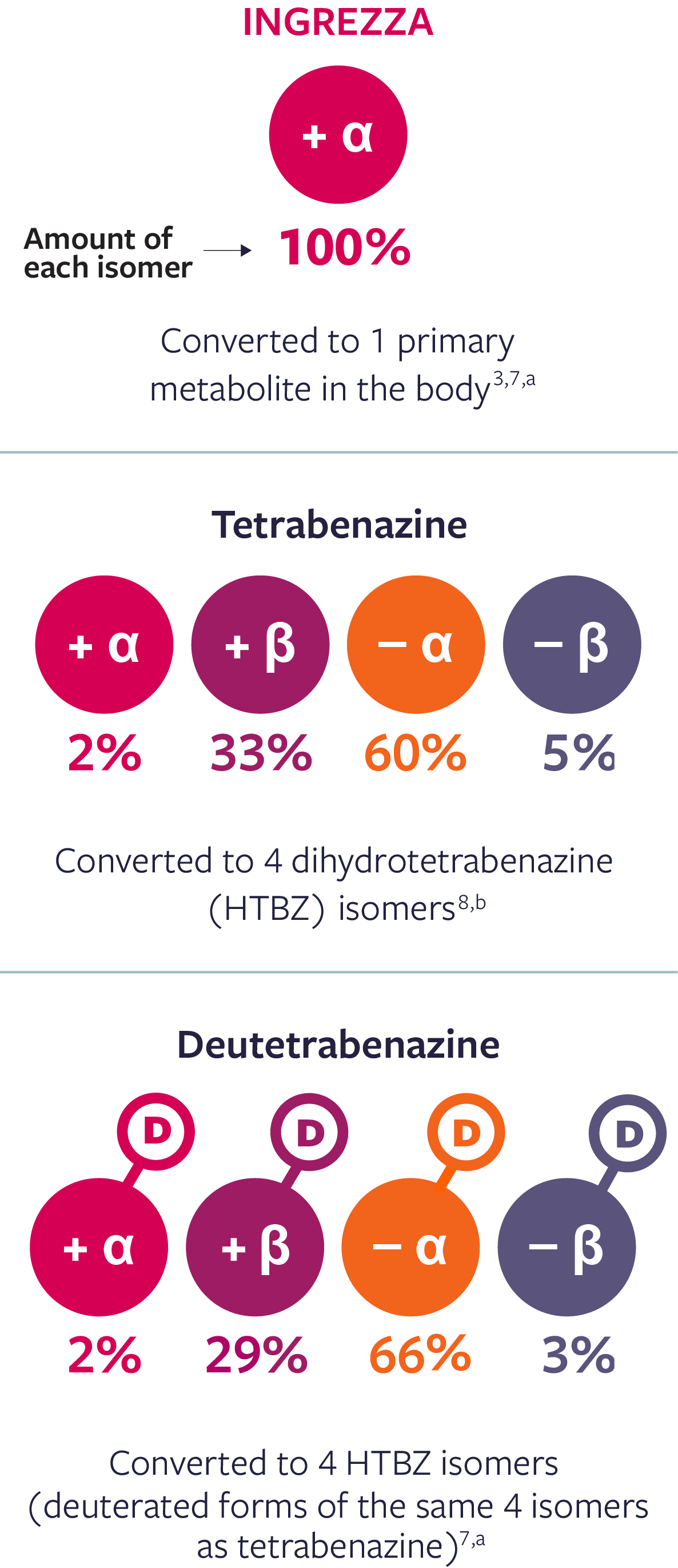
| a | Concentrations of INGREZZA and its + α metabolite and each of the 4 deutetrabenazine metabolites were assessed in a single-center, phase 1, open-label crossover study following single-dose |
| b | Concentrations of tetrabenazine isomers were determined in serum sample from 1 subject taking tetrabenazine 25 mg that was purchased from a commercial specimen bank.8 |
Based on in vitro VMAT2 binding affinity of dihydrotetrabenazine (HTBZ) metabolites and the primary active metabolite of INGREZZA, + α HTBZ. |
The activity of these compounds is based on in vitro data and the clinical implications are unknown. These data do not imply superiority of any compound. Head-to-head trials comparing tetrabenazine and deutetrabenazine to valbenazine have not been conducted.
The primary metabolite of INGREZZA—+ α—has high affinity for VMAT2 and no appreciable
binding for off-target receptors3,7,8,10
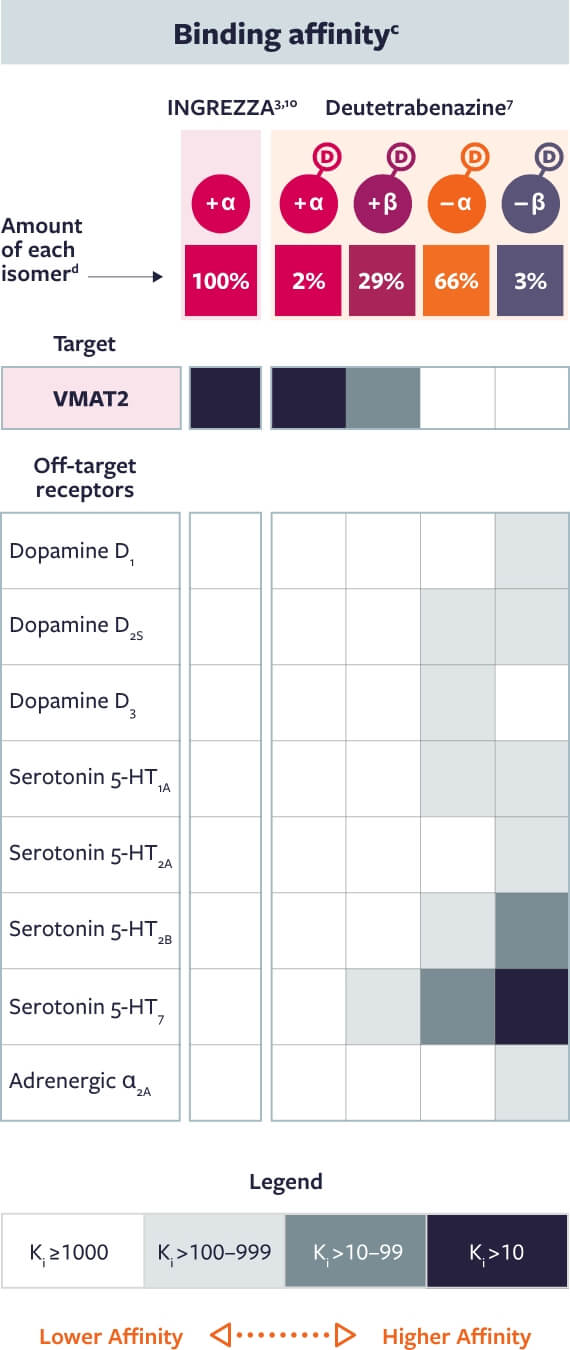
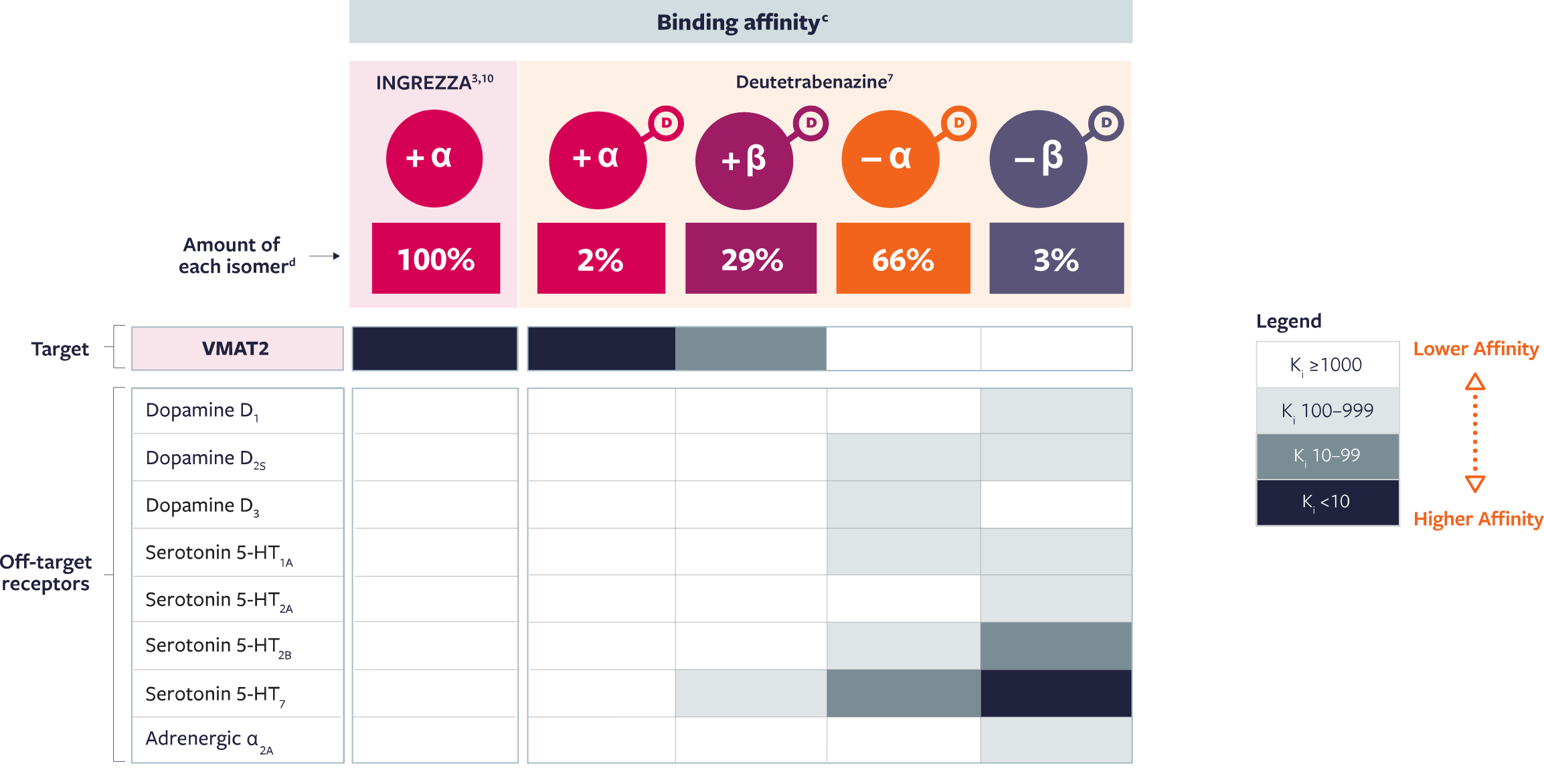
| c | Pharmacological profiles of isomers were assessed in vitro through radioligand binding assays in 2 separate studies.3,7 For the INGREZZA metabolite study, all assays were performed in 2 independent experiments and in triplicate.3 For the deuterated isomer study, all assays were performed in 1 to 3 independent experiments and in duplicate.7 |
| d | Concentrations of INGREZZA and its + α metabolite and each of the 4 deutetrabenazine metabolites were assessed in a single-center, phase 1, open-label crossover study following single-dose administration of valbenazine 40 mg or deutetrabenazine 24 mg (two 12 mg tablets) in 18 male subjects.7 |
Based on in vitro VMAT2 binding affinity of dihydrotetrabenazine (HTBZ) metabolites and the primary active metabolite of INGREZZA, + α HTBZ. |
The activity of these compounds is based on in vitro data and the clinical implications are unknown. These data do not imply superiority of any compound. Head-to-head trials comparing deutetrabenazine to valbenazine have not been conducted.
SIMPLE FROM THE START
The only VMAT2 inhibitor that offers an effective starting dosage you can adjust based on response and tolerability1
EXPLORE DOSINGTREAT FIRST LINE WITH
VMAT2 INHIBITORS
VMAT2 inhibitors, like INGREZZA, are recommended as first-line treatment for TD11-13
TD GUIDELINESREFERENCES:
- INGREZZA [package insert]. San Diego, CA: Neurocrine Biosciences, Inc.
- Harriott ND, Williams JP, Smith EB, Bozigian HP, Grigoriadis DE. VMAT2 inhibitors and the path to INGREZZA (valbenazine). Prog Med Chem. 2018;57(1):87-111.
- Grigoriadis DE, Smith E, Hoare SRJ, Madan A, Bozigian H. Pharmacologic characterization of valbenazine (NBI-98854) and its metabolites. J Pharmacol Exp Ther. 2017;361(3):454-461.
- Hauser RA, Factor SA, Marder SR, et al. KINECT 3: a phase 3 randomized, double-blind, placebo-controlled trial of valbenazine for tardive dyskinesia. Am J Psychiatry. 2017;174(5):476-484.
- Coppen EM, Roos RA. Current pharmacological approaches to reduce chorea in Huntington’s disease. Drugs. 2017;77(1):29-46.
- Vesicular monoamine transporter 2 (VMAT2) inhibitors. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Accessed March 10, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548187/?report=reader.
- Brar S, Vijan A, Scott FL, et al. Pharmacokinetic and pharmacologic characterization of the dihydrotetrabenazine isomers of deutetrabenazine and valbenazine [published online ahead of print, 2022 Dec 18]. Clin Pharmacol Drug Dev. 2022;10.1002/cpdd.1205.
- Skor H, Smith EB, Loewen G, O’Brien CF, Grigoriadis DE, Bozigian H. Differences in dihydrotetrabenazine isomer concentrations following administration of tetrabenazine and valbenazine. Drugs R D. 2017;17(3):449-459.
- Stahl SM. Comparing pharmacologic mechanism of action for the vesicular monoamine transporter 2 (VMAT2) inhibitors valbenazine and deutetrabenazine in treating tardive dyskinesia: does one have advantages over the other? CNS Spectr. 2018;23(4):239-247.
- Data on file. Neurocrine Biosciences, Inc.
- Keepers GA, Fochtmann LJ, Anzia JM, et al. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. 3rd ed. American Psychiatric Association Publishing, 2020.
- Bhidayasiri R, Jitkritsadakul O, Friedman JH, Fahn S. Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018;389:67-75.
- Caroff SN, Citrome L, Meyer J, et al. A modified Delphi consensus study of the screening, diagnosis, and treatment of tardive dyskinesia. J Clin Psychiatry. 2020;81(2):19cs12983.
